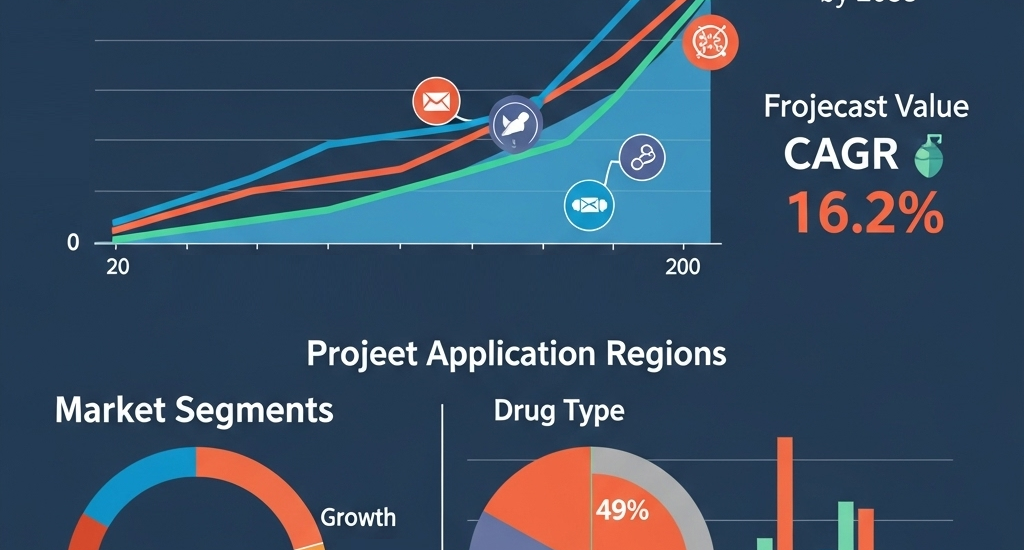
The global inhalable biologics market will witness remarkable growth, expanding from USD 4.6 billion in 2025 to nearly USD 18.9 billion by 2035, registering a CAGR of 16.2%. We’re living in an era where chronic lung diseases like asthma and COPD are becoming more common, more complex, and more expensive to treat. Yet we’re still sticking needles in people when we could be delivering treatment through the air they breathe. It’s maddening.
The idea of inhaling a biologic drug to treat serious disease sounds like science fiction. It shouldn’t. We have the science. We have the delivery devices. What we don’t have—especially in the U.S.—is the urgency to act.
Get Sample Report: – https://www.futuremarketinsights.com/reports/sample/rep-gb-22372
🏥 The U.S. Is Moving Too Slowly—And Patients Will Pay for It
Despite all our biotech bravado, the United States is trailing. FMI points out that North America is poised for growth, but that’s mostly driven by potential—not policy. There is no clear FDA framework for inhalable biologics. Each product is evaluated case-by-case. That’s bureaucratic speak for slow and uncertain.
We’ve approved inhaled therapies for decades—but only a few biologics have made it through the gauntlet. Why? Because the regulatory landscape is outdated. Because we’re afraid of innovation that doesn’t look like a pill or a syringe. And frankly, because drugmakers haven’t pushed hard enough.

🔬 The Science Is Ready. The System Is Not.
Let’s be honest—this isn’t easy science. Biologics are fragile. Transforming them into particles that can survive the journey into the lungs without falling apart is a tall order. The manufacturing process is delicate, expensive, and unforgiving.
Devices are another hurdle. Not every inhaler can deliver these drugs with the precision required. Some will need to be custom-built. That costs money. It takes time. But none of that should stop us.
As FMI highlights, the lack of a unified global regulatory framework is stalling innovation. Until the FDA, EMA, and others lay down clear, scalable pathways, the industry will continue to fumble around in the dark.
⚖️ This Is a Public Health Opportunity. We’re Wasting It.
Let’s call this what it is: a missed opportunity. Inhalable biologics aren’t just a novel drug delivery method. They are a chance to make treatment less painful, more targeted, and more accessible. No one enjoys injections. But millions endure them daily—needlessly.
If the numbers FMI reports are even half right, this is the kind of disruptive innovation that healthcare systems should be rallying behind. Not hesitating. Not gatekeeping. Certainly not ignoring.
The science is there. The market is begging. The patients are ready. Now it’s time for regulators, pharma companies, and policymakers to get serious—or get out of the way.
Explore In-Depth Analysis-Click Here to Access the Report:- https://www.futuremarketinsights.com/reports/inhalable-biologics-market
Inhalable Biologics Market Studied by Key Segments
By Product:
- Proteins and peptides, vaccines, and monoclonal antibodies.
By Application:
- Respiratory diseases, infectious diseases, and diabetes.
By Dosage Form:
- Dry powder inhalers (DPIs), metered dose inhalers (MDIs), nebulizers, and soft mist inhalers (SMIs).
By Distribution Channel:
- Hospital pharmacies, retail pharmacies, and online pharmacies.
By Region:
- North America, Europe, Asia Pacific, Latin America, and Middle East and Africa.