It starts with an orange peel. Ends with a capsule, a jam jar, or a health supplement bottle. Along the way, citrus pectin—once just a thickener for grandma’s marmalade—has quietly become a billion-dollar global commodity. But here’s what nobody wants to talk about: we’re swallowing more hype than evidence.
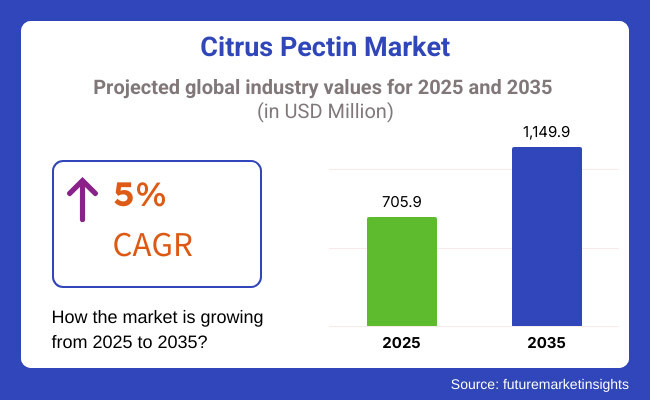
According to Future Market Insights, the citrus pectin market is projected to hit nearly $749 million in 2024, rising steadily toward a $1 billion benchmark. Food brands love it. Supplement makers swear by it. And consumers? They’re spooning it into their bodies in yogurts, gummies, pills, and powders—trusting it’s good for the heart, the gut, and maybe even cancer.
That trust is dangerously premature.
Explore Emerging Market Insights: Request a Sample Report Now: https://www.futuremarketinsights.com/reports/sample/rep-gb-11039
A Global Boom Built on Clean Labels—and Soft Claims
Let’s be clear. Pectin has real, measurable effects. As a soluble fiber, it can help lower cholesterol and slow glucose absorption when consumed in high enough quantities—around 15 grams per day. But those benefits are modest. This is not a miracle ingredient. It’s fiber. And yet, it’s being packaged and sold like some kind of medical breakthrough.
And the market is loving it.
Companies are riding the clean-label wave, hawking citrus pectin as “natural,” “gut-friendly,” “heart-healthy,” and even “anti-cancer.” These words sell. And with no FDA pre-approval required for supplements, there’s little standing in the way.
That’s not progress. That’s regulatory negligence.
High Demand for Market Insights: Discover Comprehensive Trends in Our Full Report: https://www.futuremarketinsights.com/reports/citrus-pectin-market
Modified Citrus Pectin: The Poster Child of Overpromise
The darling of this new frontier is “modified citrus pectin” (MCP). You’ll see it in detox powders, immune-boosting elixirs, and high-priced capsules. Its claims? Everything from heavy metal removal to cancer prevention. Yes, cancer.
But here’s the truth: There’s zero consensus in the scientific community that MCP actually delivers on these promises in humans. Some studies suggest it shows promise in labs or mice. Great. So do hundreds of compounds that never make it past Phase I trials.
And yet, these products are sold daily to people with serious illnesses and real desperation—armed with half-truths and asterisked disclaimers. That’s not just misleading. That’s ethically bankrupt.
The U.S. Is Asleep at the Regulatory Wheel
In the United States, citrus pectin is classified as “Generally Recognized as Safe” (GRAS). That’s fine for a fruit spread. But when that same ingredient is being sold in pill form, repackaged as a wellness solution or medical alternative, shouldn’t someone be asking harder questions?
How much is too much? What interactions could occur with medications? Are these high-dose formats stable? Are they even effective?
Crickets. Because we’ve built a supplement industry that thrives on ambiguity and soft science.
Other countries—particularly in Europe—are waking up to the need for more comprehensive review of food-derived bioactives. The U.S.? We’re still treating everything from echinacea to pectin like it’s peppermint tea.
Real Potential, But No Accountability
Let’s be fair: citrus pectin isn’t snake oil. There’s emerging research into its use in drug delivery and inflammatory disease management. One 2024 study even explored its role in reversing lung fibrosis in mice using pectin-based nanocarriers.
That’s fascinating. It’s also pre-clinical. And it does not justify marketing it as a functional cure-all to the public—especially without regulatory rigor.
We are at a crossroads. The market is maturing. Science is catching up. But the law? Still stuck in the 20th century.
Bottom Line: The Peel Is Real, But the Pitch Is Wild
Citrus pectin has a future. It might even be a powerful tool in food science and targeted therapies. But right now, it’s riding a wave of exaggerated health claims, unverified benefits, and almost no scrutiny. And that makes it a problem.
Consumers deserve clarity—not buzzwords. They need standards, not guesswork. And the FDA? It needs to get serious before another “natural” ingredient turns into the next wellness scandal.
Because if we’re going to swallow citrus pectin in everything from jam to medicine, we should at least know what it actually does—and what it absolutely doesn’t.
Key Company Profile
- Fiber Star
- Cargill Inc.
- DuPont
- Ceamsa
- Naturex SA
- CP Kelco
- Yantai Andre Pectin Co. Ltd.
- Herbstreith & Fox KG Pektin-Fabriken
- Herbafood Ingredients GmbH
- Silvateam S.p.A.
 Explore Functional Food Ingredients Industry Analysis: https://www.futuremarketinsights.com/industry-analysis/functional-food-ingredients
Explore Functional Food Ingredients Industry Analysis: https://www.futuremarketinsights.com/industry-analysis/functional-food-ingredients
Key Segmentation
By Product Type:
- High Methoxyl Pectin
- Low Methoxyl Pectin
By Source:
- Oranges
- Tangerines/Mandarins
- Grapefruit
- Lemon and Lime
By Application:
- Jams & Jellies
- Beverages
- Bakery Fillings & Toppings
- Dairy Products & Frozen Desserts
- Confectionery
- Meat & Poultry
- Dietary Supplements
- Functional Food
- Pharmaceutical
- Personal Care & Cosmetics
- Others
By Region:
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia Pacific
- Middle East and Africa




