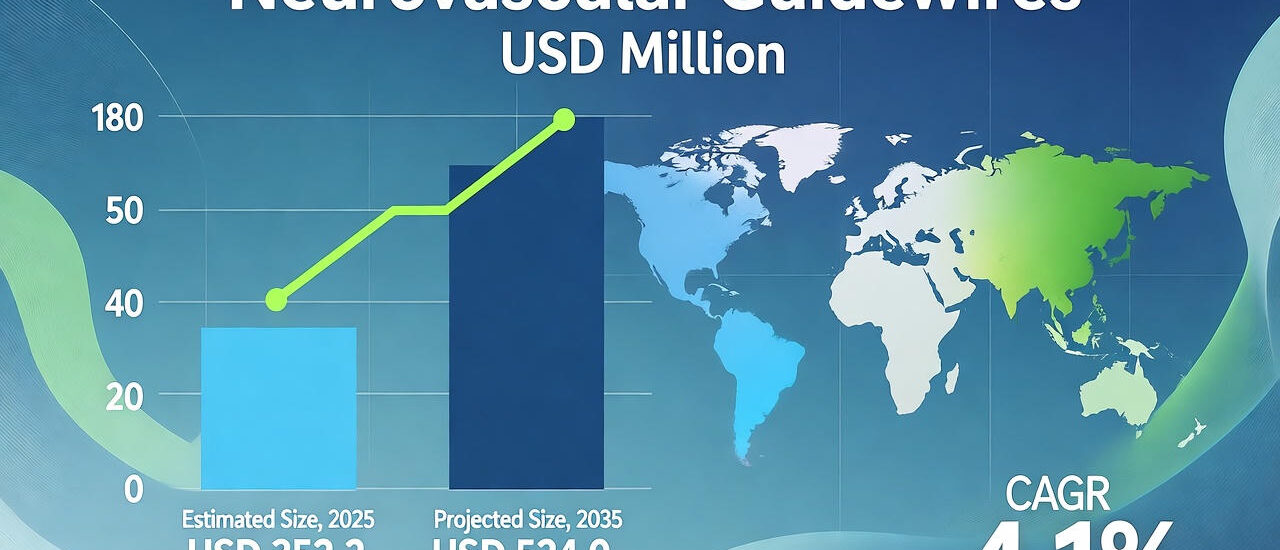
The neurovascular guidewires market is entering a transformative decade, fueled by the rising prevalence of neurovascular disorders, expanding healthcare infrastructure, and the global shift toward minimally invasive surgeries. Valued at USD 352.2 million in 2025, the market is projected to reach USD 524.0 million by 2035, growing at a CAGR of 4.1%.
Neurovascular guidewires—thin, flexible wires used to navigate delicate brain and spinal vessels—are essential tools in treating aneurysms, strokes, and arteriovenous malformations. Constructed with advanced materials such as nitinol and stainless steel, and coated with hydrophilic or hydrophobic layers, these devices minimize friction, allowing safer and more precise interventions.
Get this Report at $5000 Only (Report price) | Exclusive Discount Inside!: https://www.futuremarketinsights.com/reports/sample/rep-gb-3121
Advancing Minimally Invasive Procedures
The global healthcare community continues to prioritize minimally invasive neurovascular interventions, a field that has revolutionized how complex brain and spine disorders are treated. Procedures using guidewires enable physicians to navigate intricate vascular structures with accuracy while reducing recovery time, hospital stays, and surgical risks.
This shift is particularly pronounced in markets such as Germany, where healthcare systems emphasize procedural safety and efficiency, and in the United States, where ambulatory surgical centers (ASCs) are rapidly expanding to support outpatient neurovascular interventions. Meanwhile, China’s investment in rural healthcare infrastructure has opened access to advanced treatments, boosting demand for precision medical tools like guidewires.
Technological Innovation and Market Dynamics
Innovation remains at the heart of the neurovascular guidewires industry. The development of hydrophilic-coated guidewires, projected to account for 55.3% of the 2025 market share, has improved navigation through complex vascular pathways by reducing friction and vessel trauma. Similarly, shapeable tip guidewires, estimated to hold 35.7% of the market, offer unmatched flexibility and control during intricate procedures.
Leading global manufacturers such as Stryker, Medtronic, and Boston Scientific Corporation continue to strengthen their portfolios through R&D investments, material innovations, and strategic collaborations. In contrast, emerging companies like Baylis Medical Technologies and Scientia Vascular are entering the competitive landscape with breakthrough products and FDA-approved technologies that challenge established norms.
In May 2024, Scientia Vascular gained FDA clearance for its Plato 17 and Socrates 38 catheters for ischemic stroke treatment. In March 2024, Baylis Medical launched its PowerWire Pro RF guidewire in the United States, marking a new era of multifunctional devices that blend navigation and therapy.
Growth Drivers and Opportunities
The rising incidence of stroke, aneurysm, and AVM cases, along with aging populations and lifestyle-related conditions, continues to drive market expansion. Awareness campaigns promoting early detection and intervention have significantly increased the number of patients opting for minimally invasive treatments.
The next wave of growth is expected from multi-functional guidewires, combining navigation, drug delivery, and clot removal in one device. Such innovations not only reduce procedural complexity but also improve patient outcomes by enabling real-time, targeted therapy—crucial in time-sensitive neurovascular emergencies.
Market Challenges
While technological advancements propel the market forward, the high cost of advanced guidewires remains a barrier, particularly in cost-sensitive regions. Hospitals in developing economies often face budget constraints, limiting the adoption of premium-grade nitinol or hydrophilic-coated guidewires. This affordability gap presents an opportunity for new manufacturers to develop cost-effective yet high-performance alternatives.
Buy Now Report Here: https://www.futuremarketinsights.com/checkout/3121
Competitive Landscape and Future Outlook
Tier 1 companies currently hold over 52% of the global market, leveraging scale, reputation, and strong R&D pipelines. Tier 2 and Tier 3 players, including B. Braun, Cook Medical, Smith+Nephew, Integra, and Terumo Corporation, are increasingly competitive in niche applications and regional markets. These companies are expected to capitalize on the growing preference for precision-based, minimally invasive neurovascular procedures across emerging economies.
As the market continues to evolve, manufacturers—both established leaders and ambitious newcomers—are investing in innovation, partnerships, and geographic expansion to meet the escalating demand for advanced neurovascular tools. With continued emphasis on safety, speed, and procedural precision, the global neurovascular guidewires industry is set to play a critical role in shaping the next generation of neurological care.
Related Reports:
Community-Acquired Bacterial Pneumonia (CABP) Treatment Market- https://www.futuremarketinsights.com/reports/community-acquired-bacterial-pneumonia-treatment-market
Chlamydia Diagnostics Market- https://www.futuremarketinsights.com/reports/chlamydia-diagnostics-market
Optic Neuropathy Management Market- https://www.futuremarketinsights.com/reports/optic-neuropathy-management-market
About Future Market Insights (FMI)
Future Market Insights, Inc. (ESOMAR certified, recipient of the Stevie Award, and a member of the Greater New York Chamber of Commerce) offers profound insights into the driving factors that are boosting demand in the market. FMI stands as the leading global provider of market intelligence, advisory services, consulting, and events for the Packaging, Food and Beverage, Consumer Technology, Healthcare, Industrial, and Chemicals markets. With a vast team of over 400 analysts worldwide, FMI provides global, regional, and local expertise on diverse domains and industry trends across more than 110 countries.
Join us as we commemorate 10 years of delivering trusted market insights. Reflecting on a decade of achievements, we continue to lead with integrity, innovation, and expertise.
Contact Us:
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware – 19713, USA
T: +1-347-918-3531
For Sales Enquiries: sales@futuremarketinsights.com
Website: https://www.futuremarketinsights.com
LinkedIn| Twitter| Blogs | YouTube